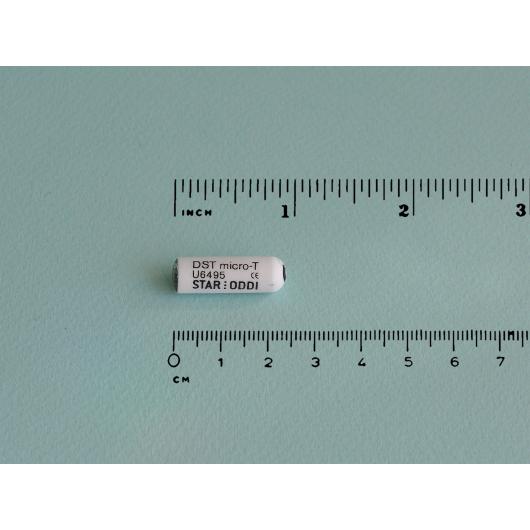
Logger for temperature measurements, implantable, micro
Key features
- Ideal for biomedical and animal welfare studies
- Reliable, constant and accurate measurements
- Simple to use and cost effective
- Multiple animals in a cage (biomedical studies)
- Bicompatible housing with dimensions 25,4 mm x 8,3 mm
Description
DST micro-T is a very small thermo logger. It measures and records temperature automatically with a customer defined interval. Data from the temperature probe is stored in the logger‘s internal memory with a real-time clock reference for each measurement and is retrieved after the study using a Communication Box.
The temperature monitor can store up to 43 477 temperature measurements and has a battery life of circa 18 months (with sampling interval of 10 min). DST micro-T logger is easy to sterilize (gas sterilizer or 70% ethanol) and can be reused as long as the battery lasts, which makes the loggers very cost efficient. Each DST micro-T has its own five digit serial number permanently marked on the logger housing as well as placed in the logger's memory and provided with all downloaded data. DST micro-T is especially useful when you wish to record a comprehensive data set throughout your research with no disturbance to the animal or subject.
The DST micro-T is supported by the Mercury (or SeaStar) software and the Communication Box which serves as an interface between the logger and a PC computer. Communication between the logger and the Communication Box is wireless when logger is placed in the Communication Box.
In the software, the user programs the start time, start date and sampling interval before the logger is implanted/deplolyed. A single interval is defined in seconds, minutes and hours. Optionally up to seven different intervals can be used within the same measurement sequence. The user defines the number of measurements they wish to record with each set interval at one time and the order these intervals are repeated in. This is especially useful when more frequent or rare measurements are needed at certain time periods.
After recovering the DST, recorded data is uploaded in the software where the results are displayed both in graphic and tabular form. The software also provides the user with some basic statistic information on the data such as minimum and maximum values on defined area, median, average, distribution of values etc. When recorded data has been retrieved, the DST can be re-programmed and reused as long as the battery lasts.
A set of Communication Box and Mercury (or SeaStar) software needs to be purchased with the first order.
- Sensor: temperature
- Size (diameter x length): 8.3 mm x 25.4mm
- Housing material: alumina (ceramic) and biocompatible epoxy
- Weight (in air/in water): 3.3g/1.9g
- Data resolution: 16 bit
- Temperature range: 5 to 45°c (41°f to 113°f)**
- Temperature resolution: 0.003°c (0.0054°f)
- Temperature accuracy: +/- 0.06°c (+/- 0.11°f)
- Temperature response time: time constant (63%) reached in 8 sec.
- Memory type: non-volatile eeprom
- Memory capacity: 65,535 measurements
- Sampling interval: user specified in second(s), minute(s), or hour(s)
- Minimum measuring interval: 1 second
- Multiple intervals option: up to 7 different intervals
- Data retention: 25 years
- Clock: real time clock. Accuracy +/-1 min/month
- Communications: communication box, wirelss transmission when dst sits in the box. Connection to pc: usb cable
- Attachment hole: 0.5 mm (diameter)
- Battery life: 28 months*
- Replaceable battery: no
* for sampling interval of 10 min.
** outside ranges available upon request.
Specifications may change without notice.
- COMBOX Communication box with USB converter
- MERCURY Mercury software for DST system
- SeaStar SeaStar software for DST system - Graphic supporting software for Windows
2015
Ballou MA, Hanson DL, Cobb CJ, Obeidat BS, Sellers MD, Pepper-Yowell AR, Carroll JA, Earleywine TJ and Lawhon SD.
Plane of nutrition influences the performance, innate leukocyte responses, and resistance to an oral Salmonella enterica serotype Typhimurium challenge in Jersey calves.
Journal of Dairy Science (2015) March; 98(3); 1972-1982.
2014
Isakova-Sivak I, de Jonge J, Smolonogina T, Rekstin A, van Amerongen G, et al.
Development and Pre-Clinical Evaluation of Two LAIV Strains against Potentially Pandemic H2N2 Influenza Virus.
PLoS ONE (2014) July; 9(7): e102339.
Mann AJ, Noulin N, Catchpole A, Stittelaar KJ, de Waal L, Veldhuis Kroeze EJB, Hinchcliffe M, Smith A, Montomoli E, Piccirella S, Osterhaus ADME, Knight A, Oxford JS, Lapini G, Cox R, Lambkin-Williams R.
Intranasal H5N1 Vaccines, Adjuvanted with Chitosan Derivatives, Protect Ferrets against Highly Pathogenic Influenza Intranasal and Intratracheal Challange.
PLoS One (2014) May; 9(5): e93761.
van Geest L, Keehnen M, Klomp R, Bakker J, van der Schilt R and Langermans J.
A simple solution to prevent the abdominal migration of temperature loggers, and to facilitate their smooth retrieval post-study in macaques.
Biomedical Primate Research Centre.
Maltais A-K, Stittelaar KJ, Vedhuis K, van Amerongen G, Dijkshoorn ML, Krestin GP, Hinkula J, Arwidsson H, Lindberg A, Osterhaus ADME.
Intranasally administered Endocine™ formulated 2009 pandemic influenza H1N1 vaccine induces broad specific antibody responses and confers protection in ferrets.
Vaccine (2014) May 30; 32(26): 3307-3315.
Geiser B, Burfeind O, Heuwieser W, Arlt S.
Prediction of Parturition in Bitches Utilizing Continuous Vaginal Temperature Measurement.
Reproduction in Domestic Animals (2014) February, 49(1):109-114.
Hynd PI, Czerwinski VH, McWhorter TJ.
Is propensity to obesity associated with the diurnal pattern of core body temperature?
International Journal of Obesity (2014) 38:231-235.
2013
van den Brand J, Helgadóttir B, Stittelaar K, Bjarnason Á, de Waal L, de Swart R, Kuiken T, Osterhaus A.
The use of Star-Oddi temperature loggers in laboratory animal experiments for pathogenesis research and evaluation of prevention and treatment of infectious diseases.
Presented at the 13th Annual Meeting of Safety Pharmacology Society (SPS) September 16-19, 2013.
van der Vries E, Stittelaar KJ, van Amerongen G, Vedhuis Kroeze EJB, de Waal L, Fraaij PLA, Meesters RJ, Luider TM, van der Nagel B, Koch B, Vulto AG, Schutten M, Osterhaus ADME.
Prolonged Influenza Virus Shedding and Emergence of Antiviral Resistance in Immunocompromised Patients and Ferrets.
PLoS Pathogens (2013) May; 9(5): e1003343.
Suthar V, Burfeind O, Maeder B, Heuwieser W.
Agreement between rectal and vaginal temperature measured with temperature loggers in dairy cows.
Journal of Dairy Research (2013) May; 80 (2) : 240-245.
Maeder B., Arlt S., Burfeind O. and Heuwieser W.
Continuous vaginal temperature measurement in bitches before parturition.
Reproductive Biology (2013) Feb; Vol 13 (Suppl. 2), 31-32.
Download poster
van den Brand JMA, Doctoral Thesis.
Experimental SARS and influenza: similar disease, different pathways.
Bodewes R, Kreijtz JHCM., van Amerongen G, Hillaire MLB, Vogelzang-van Trierum SE, Nieuwkoop NJ, van Run P, Kuiken T, Fouchier RAM, Osterhaus ADME and Rimmelzwaan GF.
Infection of the upper respiratory tract with seasonal influenza A(H3N2) virus induces protective immunity in ferrets against infection with A(H1N1)pdm09 virus after intranasal, but not intratracheal inoculation.
Journal of Virology (2013) Feb; 87(4).
Dias WO, van der ARK AAJ, Sakauchi MA, Kubrusly FS, Prestes AFRO, Borges MM, Furuyama N, Horton DSPQ, Quintilio W, Antoniazi M, Kuipers B, van der Zeijst BAM, Raw I.
An improved whole cell pertussis vaccine with reduced content of endotoxin.
Human Vaccines & Immunotherapeutics (2013) Feb; 9(2): 1–10.
2012
Maeder B, Arlt S, Burfeind O and Heuwieser W.
Application of Vaginal Temperature Measurement in Bitches.
Reprod Dom Anim (2012) Dec; 47 (Suppl. 6), 1–3.
Download poster
Edwin J.B, Kroeze V, Kuiken T. Osterhaus A.D.M.E.
Animal Models.
Influenza Virus. (Methods in Molecular Biology, vol. 865, 127-146). Humana Press.
van den Brand JMA, Stittelaar KJ, van Amerongen G, Reperant L, de Waal L, et al.
Comparison of Temporal and Spatial Dynamics of Seasonal H3N2, Pandemic H1N1 and Highly Pathogenic Avian Influenza H5N1 Virus Infections in Ferrets.
PLoS ONE (2012) Aug; 7(8): e42343.
Kaaijk P, van der Ark AAJ, van Amerongen G, van den Dobbelsteen GPJM.
Nonclinical vaccine safety evaluation: advantages of continuous temperature monitoring using abdominally implanted data loggers.
J. Appl. Toxicol. (2012).
Burdick NC, Carroll JA, Dailey JW, Randel RD, Falkenberg SM, Schmidt TB.
Development of a self-contained, indwelling vaginal temperature probe for use in cattle research.
Journal of Thermal Biology (2012); 37;339–343.
van den Brand JMA, Stittelaar KJ, Leijten LM, van Amerongen G, Simon JH, Osterhaus AD, Kuiken T.
Modification of the ferret model for pneumonia from seasonal human influenza A virus infection.
Veterinary Pathology May 2012 vol. 49 no. 3 562-568.
2011
Stittelaar KJ, Veldhuis Kroeze EJ, Rudenko L, Dhere R, Thirapakpoomanunt S, Kieny MP, Osterhaus AD.
Efficacy of live attenuated vaccines against 2009 pandemic H1N1 influenza in ferrets.
Vaccine (2011) Nov 15;29(49):9265-70. Epub 2011 Sep 22.
Ballou MA, Cobb CJ, Hulbert LE, Carroll JA.
Effects of intravenous Escherichia coli dose on the pathophysiological response of colostrum-fed Jersey calves.
Veterinary Immunology and Immunopathology. (2011); 141; 76–83.
van den Brand JMA, Kreijtz JH, Bodewes R, Stittelaar KJ, van Amerongen G, Kuiken T, Simon J, Fouchier RA, Del Giudice G, Rappuoli R, Rimmelzwaan GF, Osterhaus AD.
Efficacy of vaccination with different combinations of MF59-adjuvanted and nonadjuvanted seasonal and pandemic influenza vaccines against pandemic H1N1 (2009) influenza virus infection in ferrets.
J Virol. 2011 Mar;85(6):2851-8. Epub 2011 Jan 5.
Baras B, Stittelaar KJ, Kuiken T, Jacob V, Bernhard R, Giannini S, de Waal L, van Amerongen G, Simon JH, Osterhaus AD, Hanon E, Mossman SP.
Longevity of the protective immune response induced after vaccination with one or two doses of AS03A-adjuvanted split H5N1 vaccine in ferrets.
Vaccine (2011) Mar 3;29(11):2092-9. Epub 2011 Jan 13.
2010
Herfst S, van den Brand JMA, Schrauwen EJA, de Wit E., Munster VJ, van Amerongen G., Linster M, Zaaraoui F., van Ijcken WFJ, Rimmelzwaan GF, Osterhaus ADME, Fouchier RAM, Andeweg AC, Kuiken T.
Pandemic 2009 H1N1 Influenza Virus Causes Diffuse Alveolar Damage in Cynomolgus Macaques.
Vet Pathol (2010); 47:1040-1047.
Hamelin M-E, Baz M, Abed Y, Couture C, Joubert P, Beaulieu E, Bellerose N, Plante M, Mallett C, Schumer G, Kobinger GP, Boivin G.
Oseltamivir-Resistant Pandemic A/H1N1 Virus Is as Virulent as Its Wild-Type Counterpart in Mice and Ferrets.
PLoS Pathog. (2010); 6(7): e1001015.
Friesen RHE, Koudstaal W, Koldijk MH, Weverling GJ, Brakenhoff JPJ, et al.
New Class of Monoclonal Antibodies against Severe Influenza: Prophylactic and Therapeutic Efficacy in Ferrets.
PLoS ONE (2010) Feb; 5(2): e9106
van den Brand JMA, Stittelaar KJ, van Amerongen G, Rimmelzwaan GF, Simon J,de Wit E, Munster V, Bestebroer T, Fouchier R.A.M, Kuiken T, Osterhaus A.D.M.E.
Severity of Pneumonia Due to New H1N1 Influenza Virus in Ferrets Is Intermediate between That Due to Seasonal H1N1 Virus and Highly Pathogenic Avian Influenza H5N1 Virus.
J Infect Dis. (2010); 201 (7): 993-999.
Download poster
Stittelaar KJ, Lacombe V, van Lavieren R, van Amerongen G, Simon J, Cozette V, Swayne DE, Poulet H, Osterhaus ADME.
Cross-clade immunity in cats vaccinated with a canarypox-vectored avian influenza vaccine.
Vaccine 28. (2010); 4970–4976.
2009
Kreijtz JHCM, Suezer Y, de Mutsert G, van den Brand MA, van Amerongen G, Schnierle BS, Kuiken T, Fouchier RAM, Osterhaus ADME, Sutter G, Rimmelzwaan GF.
Recombinant Modified Vaccinia Virus Ankara Expressing the Hemagglutinin Gene Confers Protection against Homologous and Heterologous H5N1 Influenza Virus Infections in Macaques.
J Infect Dis. (2009); 199 (3): 405-413.
2008
Reperant LA, van Amerongen G, van de Bildt MWG, Rimmelzwaan GF, Dobson AP, Osterhaus ADME, Kuiken T.
Highly Pathogenic Avian Influenza Virus (H5N1) Infection in Red Foxes Fed Infected Bird Carcasses.
Emerging Infectious Diseases (2008); 14 (12).