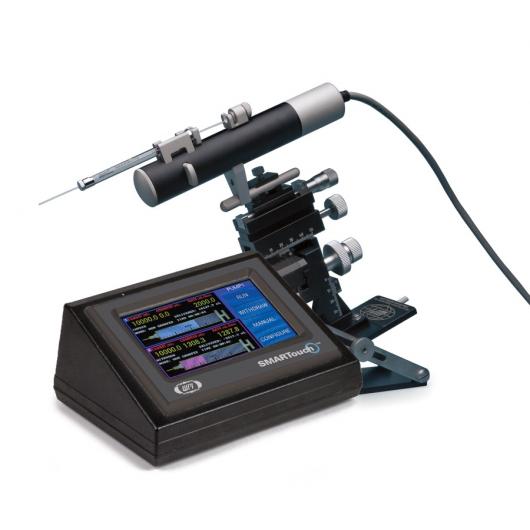
Microinjection syringe pump
The UMP3 (UltraMicroPump3) is a versatile microinjection syringe pump paired with microsyringes to deliver microliter-level volumes. The UMP3 is optimal for applications requiring precise, targeted injections of extremely low-volume samples. With its touchscreen controller, our UMP3 can deliver or withdraw as little as 25nL using 10µL syringe with 60 mm stroke length.
- Graphic display with SMARTouch™ touch screen controller for "intelligent", easy to use interface controlling up to four microliter syringe pumps
- Splash proof touch screen
- User configurable mounting bar
- Dual mode motor drive
- Compatible with all UMP, UMP2 and UMP3 micro syringe pumps
- Optional foot switch available
- 5 digit display
Benefits
- Accepts a wide range of microinjection syringes from 0.5 ul to 1000 ul.
- Manual or automated injections
- Quiet operation for electrophysiology recordings
- Mounts directly on micromanipulator or stereotaxic frame
- Nominal injections down to 1 nL
- Rapid setup with intuitive touchscreen controller
Applications
- Microinjection
- Neuroscience
- Microfluidics
- Micro delivery of biochemical agents or dyes
- Intravitreal Injection (with RPE-KIT)
- Intraoccular Injection (with IO-KIT)
Zhou, Z., Luther, N., Singh, R., Boockvar, J. A., Souweidane, M. M., & Greenfield, J. P. (2017). Glioblastoma spheroids produce infiltrative gliomas in the rat brainstem.
Child’s Nervous System, 1–10. http://doi.org/10.1007/s00381-017-3344-y
Ye, H.-L., Li, D.-R., Yang, J.-S., Chen, D.-F., De Vos, S., Vuylsteke, M., … Yang, W.-J. (2017). Molecular characterization and functional analyses of a diapause hormone receptor-like gene in parthenogenetic Artemia.
Peptides. http://doi.org/10.1016/j.peptides.2017.01.008
Wofford, K. L., Harris, J. P., Browne, K. D., Brown, D. P., Grovola, M. R., Mietus, C. J., … Cullen, D. K. (2017). Rapid neuroinflammatory response localized to injured neurons after diffuse traumatic brain injury in swine.
Experimental Neurology, 290, 85–94. http://doi.org/10.1016/j.expneurol.2017.01.004
Qi, Y., Purtell, L., Fu, M., Zhang, L., Zolotukhin, S., Campbell, L., & Herzog, H. (2017). Hypothalamus specific re-introduction of Snord116 into otherwise Snord116 deficient mice increased energy expenditure.
Journal of Neuroendocrinology. http://doi.org/10.1111/jne.12457
Mosberger, A. C., Miehlbradt, J. C., Bjelopoljak, N., Schneider, M. P., Wahl, A.-S., Ineichen, B. V., … Schwab, M. E. (2017). Axotomized Corticospinal Neurons Increase Supra-Lesional Innervation and Remain Crucial for Skilled Reaching after Bilateral Pyramidotomy.
Cerebral Cortex, 137, 1716–1732. http://doi.org/10.1093/cercor/bhw405
Job, M. O., & Kuhar, M. J. (2017). CART peptide in the nucleus accumbens regulates psychostimulants: Correlations between psychostimulant and CART peptide effects.
Neuroscience, 348, 135–142. http://doi.org/10.1016/j.neuroscience.2017.02.012
Eleftheriadou, I., Dieringer, M., Poh, X. Y., Sanchez-Garrido, J., Gao, Y., Sgourou, A., … Mazarakis, N. D. (2017). Selective transduction of astrocytic and neuronal CNS subpopulations by lentiviral vectors pseudotyped with Chikungunya virus envelope.
Biomaterials, 123, 1–14. http://doi.org/10.1016/j.biomaterials.2017.01.023
Augestad, I. L., Nyman, A. K. G., Costa, A. I., Barnett, S. C., Sandvig, A., Håberg, A. K., & Sandvig, I. (2017). Effects of Neural Stem Cell and Olfactory Ensheathing Cell Co-transplants on Tissue Remodelling After Transient Focal Cerebral Ischemia in the Adult Rat.
Neurochemical Research, 1–11. http://doi.org/10.1007/s11064-016-2098-3
Lin, P., Fang, Z., Liu, J., & Lee, J. H. (2016). Optogenetic Functional MRI.
Journal of Visualized Experiments, (110), e53346–e53346. http://doi.org/10.3791/53346
Vacca, O., El Mathari, B., Darche, M., Sahel, J.-A., Rendon, A., & Dalkara, D. (2015). Using Adeno-associated Virus as a Tool to Study Retinal Barriers in Disease.
Journal of Visualized Experiments, (98), e52451–e52451. http://doi.org/10.3791/52451
Lai, J., Legault, M.-A., Thomas, S., & Casanova, C. (2015). Simultaneous Electrophysiological Recording and Micro-injections of Inhibitory Agents in the Rodent Brain.
Journal of Visualized Experiments, (101), e52271–e52271. http://doi.org/10.3791/52271
Robinson, S., & Adelman, J. S. (2015). A Method for Remotely Silencing Neural Activity in Rodents During Discrete Phases of Learning.
Journal of Visualized Experiments, (100), e52859–e52859. http://doi.org/10.3791/52859
Platt, R. J., Chen, S., Zhou, Y., Yim, M. J., Swiech, L., Kempton, H. R., … Zhang, F. (2014). CRISPR-Cas9 Knockin Mice for Genome Editing and Cancer Modeling.
Cell, 159(2), 440–55. http://doi.org/10.1016/j.cell.2014.09.014
Pierce, A. M., & Keating, A. K. (2014). Creating Anatomically Accurate and Reproducible Intracranial Xenografts of Human Brain Tumors.
Journal of Visualized Experiments, (91), e52017–e52017. http://doi.org/10.3791/52017
Paveliev, M., Kislin, M., Molotkov, D., Yuryev, M., Rauvala, H., & Khiroug, L. (2014). Acute Brain Trauma in Mice Followed By Longitudinal Two-photon Imaging.
Journal of Visualized Experiments?: JoVE, (April), 1–8. http://doi.org/10.3791/51559
Nakamura, S., Baratta, M. V., & Cooper, D. C. (2013). A Method for High Fidelity Optogenetic Control of Individual Pyramidal Neurons
In vivo Journal of Visualized Experiments, (79), e50291–e50291. http://doi.org/10.3791/50291
Inquimbert, P., Moll, M., Kohno, T., & Scholz, J. (2013). Stereotaxic Injection of a Viral Vector for Conditional Gene Manipulation in the Mouse Spinal Cord.
Journal of Visualized Experiments, (73), e50313–e50313. http://doi.org/10.3791/50313
Hewing, N. J., Weskamp, G., Vermaat, J., Farage, E., Glomski, K., Swendeman, S., … Blobel, C. P. (2013). Intravitreal injection of TIMP3 or the EGFR inhibitor erlotinib offers protection from oxygen-induced retinopathy in mice.
Investigative Ophthalmology & Visual Science, 54(1), 864–70. http://doi.org/10.1167/iovs.12-10954
Salt, A. N., Hartsock, J. J., Gill, R. M., Piu, F., & Plontke, S. K. (2012). Perilymph Pharmacokinetics of Markers and Dexamethasone Applied and Sampled at the Lateral Semi-Circular Canal.
Journal of the Association for Research in Otolaryngology, 13(6), 771–783. http://doi.org/10.1007/s10162-012-0347-y
Nickerson, J. M., Goodman, P., Chrenek, M. A., Bernal, C. J., Berglin, L., Redmond, T. M., & Boatright, J. H. (2012). Subretinal delivery and electroporation in pigmented and nonpigmented adult mouse eyes.
Methods in Molecular Biology (Clifton, N.J.), 884, 53–69. http://doi.org/10.1007/978-1-61779-848-1_4
Beier, K., & Cepko, C. (2012). Viral Tracing of Genetically Defined Neural Circuitry.
Journal of Visualized Experiments, (68), e4253–e4253. http://doi.org/10.3791/4253
Goel, M., Sienkiewicz, A. E., Picciani, R., Wang, J., Lee, R. K., & Bhattacharya, S. K. (2012). Cochlin, intraocular pressure regulation and mechanosensing.
PloS One, 7(4), e34309. http://doi.org/10.1371/journal.pone.0034309
Abdelwahab, M. G., Sankar, T., Preul, M. C., & Scheck, A. C. (2011). Intracranial Implantation with Subsequent 3D In Vivo Bioluminescent Imaging of Murine Gliomas.
Journal of Visualized Experiments, (57), e3403–e3403. http://doi.org/10.3791/3403
Lowery, R. L., & Majewska, A. K. (2010). Intracranial Injection of Adeno-associated Viral Vectors.
Journal of Visualized Experiments, (45), e2140–e2140. http://doi.org/10.3791/2140
Kinkel, M. D., Eames, S. C., Philipson, L. H., & Prince, V. E. (2010). Intraperitoneal injection into adult zebrafish.
Journal of Visualized Experiments?: JoVE, (42), e2126. http://doi.org/10.3791/2126
Molotkov, D. A., Yukin, A. Y., Afzalov, R. A., & Khiroug, L. S. (2010). Gene Delivery to Postnatal Rat Brain by Non-ventricular Plasmid Injection and Electroporation.
Journal of Visualized Experiments, (43), e2244–e2244. http://doi.org/10.3791/2244
Marker, D. F., Tremblay, M.-E., Lu, S.-M., Majewska, A. K., & Gelbard, H. A. (2010). A Thin-skull Window Technique for Chronic Two-photon In vivo Imaging of Murine Microglia in Models of Neuroinflammation.
Journal of Visualized Experiments, (43), e2059–e2059. http://doi.org/10.3791/2059
Eames, S. C., Philipson, L. H., Prince, V. E., & Kinkel, M. D. (2010). Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis.
Zebrafish, 7(2), 205–13. http://doi.org/10.1089/zeb.2009.0640
Jasnow, A. M., Rainnie, D. G., Maguschak, K. A., Chhatwal, J. P., & Ressler, K. J. (2009). Construction of Cell-Type Specific Promoter Lentiviruses for Optically Guiding Electrophysiological Recordings and for Targeted Gene Delivery (pp. 199–213).
http://doi.org/10.1007/978-1-59745-559-6_13
Christiana J. Johnson, Lennart Berglin, Micah A. Chrenek, T.M. Redmond, Jeffrey H. Boatright, J. M. N. (2008). Technical Brief: Subretinal injection and electroporation into adult mouse eyes.
Molecular Vission, 14, 2211–2226. Retrieved from http://www.molvis.org/molvis/v14/a259/
Takayama, K., Torashima, T., Horiuchi, H., & Hirai, H. (2008). Purkinje-cell-preferential transduction by lentiviral vectors with the murine stem cell virus promoter. Neuroscience Letters (Vol. 443).
Torashima, T., Yamada, N., Itoh, M., Yamamoto, A., & Hirai, H. (2006). Exposure of lentiviral vectors to subneutral pH shifts the tropism from Purkinje cell to Bergmann glia.
European Journal of Neuroscience, 24(2), 371–380. http://doi.org/10.1111/j.1460-9568.2006.04927.x
Torashima, T., Okoyama, S., Nishizaki, T., & Hirai, H. (2006). In vivo transduction of murine cerebellar Purkinje cells by HIV-derived lentiviral vectors.
Brain Research, 1082(1), 11–22. http://doi.org/10.1016/j.brainres.2006.01.104
Dancause, N., Barbay, S., Frost, S. B., Plautz, E. J., Chen, D., Zoubina, E. V, … Nudo, R. J. (n.d.) (2005). Development/Plasticity/Repair Extensive Cortical Rewiring after Brain Injury.
https://doi.org/10.1523/JNEUROSCI.3256-05.2005
Cherezov, V., Peddi, A., Muthusubramaniam, L., Zheng, Y. F., & Caffrey, M. (2004). A robotic system for crystallizing membrane and soluble proteins in lipidic mesophases.
Acta Crystallographica Section D Biological Crystallography, 60(10), 1795–1807. http://doi.org/10.1107/S0907444904019109
Bernd, A. S., Aihara, M., Lindsey, J. D., & Weinreb, R. N. (2004). Influence of Molecular Weight on Intracameral Dextran Movement to the Posterior Segment of the Mouse Eye.
Investigative Opthalmology & Visual Science, 45(2), 480. http://doi.org/10.1167/iovs.03-0462
Shawgo, R. S. (2004). In vivo activation and biocompatibility of a MEMS microreservoir drug delivery device.
Retrieved November 16, 2016, from http://citeweb.info/20041104095
Nelson, B. P., Grimsrud, T. E., Liles, M. R., Goodman, R. M., & Corn, R. M. (n.d.) (2001). Surface Plasmon Resonance Imaging Measurements of DNA and RNA Hybridization Adsorption onto DNA Microarrays.
https://doi.org/10.1021/ac0010431
Sturbaum, G. D., Reed, C., Hoover, P. J., Jost, B. H., Marshall, M. M., & Sterling, C. R. (2001). Species-Specific, Nested PCR-Restriction Fragment Length Polymorphism Detection of Single Cryptosporidium parvum Oocysts.
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, 67(6), 2665–2668. https://www.ncbi.nlm.nih.gov/pubmed/11375178
| Product name | |
|---|---|
| UMP3T-1 Microinjection Syringe Pump, UltraMicroPump3 (one) and SMARTouch Controller | |
| UMP3T-2 Microinjection Syringe Pump, UltraMicroPump3 (two) and SMARTouch Controller | |
| UMP3 Microinjection Syringe Pump, UltraMicroPump3 ONLY (without controller) | |
| MICRO2T Microinjection Syringe Pump, SMARTouch Controller ONLY, Two-Channel |