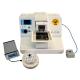

Vibratome SMZ
Campden's top of the range high precision, vibrating microtome (vibrotome for short), this is the finest tissue slicer in the world for preparations for visual patch clamping or high resolution imaging. The 7000smz series Vibrating microtome has been the instrument of choice for many labs for 10 years - over 450 labs and counting!
Key features
- Includes a z-axis calibration unit
- Z-axis blade adjust minimizer
- Blade holder angle to user requirement
- Set start and stop position from blade travel.
- Vibration speeds from 50 to 120 Hz
- Amplitudes from 0.5mm to 2.5mm
- Controlled blade advance at 10microns per sec
- Manual or automatic operation
- "Auto" programming by storage of the first slicing
- Ice water bath easily removed for cleaning
- Optional LED light and scope for clear observation
Using Campden microtomes, research detailing sectioning for visual patching of neurological tissue, heart, lung, and tissue scaffolds have all been published. The 7000smz-2 microtome represents significant advances with higher precision at a lower cost.
The vibrating mechanism of the 7000smz-2 microtome delivers unprecedented accuracy with nearly silent operation. Every machine is tested before dispatch by our in-house measurement devices and each machine is supplied with its own z-axis calibration checker.
The effect of excessive Z-axis deflection on the health and viability of the tissue preparation has been much discussed since the publication of Geiger et al (2003) and the Campden 7000smz-2 tissue slicer, with ≤ 1µm Z-axis deflection across a wide range of vibration speeds and amplitudes, delivers healthy viable sections every time.
The user interface is easy and versatile (with no foot switch unlike previous models) and offers simple operation at the push of a button or a range of changeable parameters for the expert user. The Campden 7000smz-2 also provides longevity of performance, as not only does the slicer give ≤ 1 µm performance out of the box, but the advance vibrating mechanism does not contain bearings and other elements subject to wear. Thus it will retain the ≤ 1 µm z-ad performance for years to come, giving you consistency in your biological preparation, without the need for expensive servicing.
Holding the tissue close to 4° C must continue throughout the slicing procedure. This can be done with passive cooling where a known amount of ice is used to maintain the cooled a.c.s.f. or with an electronically controlled thermo-electric Tissue Bath Cooler (7610A).
Campden developed both ceramic and stainless steel microtome blades, alongside the machine, so that the grinding and honing facets for the blade edge are matched to the blade downward angle of orientation to the tissue. The ceramic blades have a particular advantage when slicing brain tissue older than 30 days. These ceramic microtome blades also have a second advantage in that unlike stainless blades, these do not have to be replaced after each tissue sample.
Other options include an LED cold light and magnifier or scope for clear observation whilst slicing. Shown with optional Slice incubation chamber and temperature controller.
- Section thickness step size: 0.001 mm
- Bath table rise & fall speed: 1.0 mm/sec maximum
- Maximum (vertical) travel of bath table: 19 mm
- Cutting head advance speed:
- Minimum: -4.0 mm/sec (-1.00 during slicing)
- Maximum: +4.0 mm/sec (+1.00 during slicing)
- Cutting head retraction speed: 4.0 mm/sec
- Maximum travel of cutting head: 40 mm
- Blade oscillation frequency:
- Minimum: 50 Hz (lower frequenices available upon request)
- Maximum: 120 Hz (amplitude dependent)
- Frequency step size: 5 Hz
- Blade oscillation amplitude
- Minimum: 0.5 mm (nominal)
- Maximum: 2.5 mm (nominal)
- Amplitude step size: 0.25 mm (nominal)
- Power requirements (Selectable): 115VAC 60Hz, 230VAC 50Hz
- Power rating: 100W
- Fuse rating
- 115V: T2A 250VAC
- 230V: T2A 250VAC
- Light source: 100-240Vac 3W
- Dimensions: 410mm Width x 400mm Depth x 270mm Height (excluding light source and microscope)
- Weight: 33 kg (excluding microscope)
- Boxed shipping weight: 60 kg (excluding microscope)
- 7550-1-CCampden Blades, ceramic - 5 pack
- 7550-1-SSCampden Blades, stainless steel - 50 pack
- 7000-3-1ACampden Bath mount for vibrating microtomes
- 7611ACampden Temperature controlled warming tissue bath
- CL200Campden Integrally mounted cold light source
- 7000-1-3Campden Integrally mounted magnifying glass
- 7000smz-CaseCampden Case for Campden 7000 series slicer
- 7000-1-60Campden Crate for 7000 series slicer
-
Preparation of viable adult ventricular myocardial slices from large and small mammals
Samuel A Watson, Martina Scigliano, Ifigeneia Bardi, Raimondo Ascione, Cesare M Terracciano & Filippo PerbelliniNature Protocols volume 12, pages 2623-2639 (2017) - Zobacz abstrakt
-
Free-of-Acrylamide SDS-based Tissue Clearing (FASTClear) for three dimensional visualization of myocardial tissue
Filippo Perbellini, Alan K. L. Liu, Samuel A. Watson, Ifigeneia Bardi, Stephen M. Rothery & Cesare M. TerraccianoScientific Reports 7, Article number: 5188 (2017) - Zobacz abstrakt
- Integraslice Bibliography
- Isolated Living Heart Slices from Adult Rats and Guinea Pgs as a Model for Drug Testing
- MEA recordings from acute heart slices from adult rats and guinea pigs
- Rhythmicity without Synchrony in the Electrically Uncoupled Inferior Olive
- NPL consultancy on oscillating movement featured it in their “Engineering Precisely” Newsletter
- Field and Action Potential Recordings in Heart Slices: Correlation with established In Vitro and In Vivo Models
- Cardiac tissue slices with prolonged survival for in vitro drug safety screening
- Innovation in safety pharmacology testing
- Methodological innovations expand the safety pharmacology horizon
- Campden Integraslice - Development of a lung slice preparation for recording ion channel activity in alveolar epithelial type 1 cells
- Nakano, T., Nakamura, Y., Irie, K., Okano, S., Morimoto, M., Yamashita, Y., … Mishima, K. (2020). Antithrombin gamma attenuates macrophage/microglial activation and brain damage after transient focal cerebral ischemia in mice. Life Sciences, 252. https://doi.org/10.1016/j.lfs.2020.117665
- Toft-Bertelsen, T. L., Larsen, B. R., Christensen, S. K., Khandelia, H., Waagepetersen, H. S., & MacAulay, N. (2020). Clearance of activity-evoked K+ transients and associated glia cell swelling occur independently of AQP4: A study with an isoform-selective AQP4 inhibitor. GLIA. https://doi.org/10.1002/glia.23851
- Vicente, M. C., Humphrey, C. M., Gargaglioni, L. H., & Ostrowski, T. D. (2020). Decreased excitability of locus coeruleus neurons during hypercapnia is exaggerated in the streptozotocin-model of Alzheimer’s disease. Experimental Neurology, 328. https://doi.org/10.1016/j.expneurol.2020.113250
- Vicente, M. C., Humphrey, C. M., Gargaglioni, L. H., & Ostrowski, T. D. (2020). Decreased excitability of locus coeruleus neurons during hypercapnia is exaggerated in the streptozotocin-model of Alzheimer’s disease. Experimental Neurology, 328. https://doi.org/10.1016/j.expneurol.2020.113250
- Sagoshi, S., Maejima, S., Morishita, M., Takenawa, S., Otubo, A., Takanami, K., … Ogawa, S. (2020). Detection and Characterization of Estrogen Receptor Beta Expression in the Brain with Newly Developed Transgenic Mice. Neuroscience, 438, 182–197. https://doi.org/10.1016/j.neuroscience.2020.04.047
- Reisinger, S. N., Bilban, M., Stojanovic, T., Derdak, S., Yang, J., Cicvaric, A., … Pollak, D. D. (2020). Lmo3 deficiency in the mouse is associated with alterations in mood-related behaviors and a depression-biased amygdala transcriptome. Psychoneuroendocrinology, 111. https://doi.org/10.1016/j.psyneuen.2019.104480
- Brewer, C. L., Li, J., O’Conor, K., Serafin, E. K., & Baccei, M. L. (2020). Neonatal injury evokes persistent deficits in dynorphin inhibitory circuits within the adult mouse superficial dorsal horn. The Journal of Neuroscience, JN-RM- 0029-20. https://doi.org/10.1523/jneurosci.0029-20.2020
- Stojanovic, T., Benes, H., Awad, A., Bormann, D., & Monje, F. J. (2020). Nicotine abolishes memory-related synaptic strengthening and promotes synaptic depression in the neurogenic dentate gyrus of miR-132/212 knockout mice. Addiction Biology. https://doi.org/10.1111/adb.12905
- Gu, Z., Koppel, N., Kalamboglas, J., Alexandrou, G., Li, J., Craig, C., … Yoshida, Y. (2020). Semaphorin-Mediated Corticospinal Axon Elimination Depends on the Activity-Induced Bax/Bak-Caspase Pathway. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 40(28), 5402–5412. https://doi.org/10.1523/JNEUROSCI.3190-18.2020
- Khatami, L., Safari, V., & Motamedi, F. (2020). Temporary inactivation of interpeduncular nucleus impairs long but not short term plasticity in the perforant-path dentate gyrus synapses in rats. Behavioural Brain Research, 377. https://doi.org/10.1016/j.bbr.2019.112212
- Genrikhs, E. E., Stelmashook, E. V., Voronkov, D. N., Novikova, S. V., Alexandrova, O. P., Fedorov, A. V., & Isaev, N. K. (2020). The single intravenous administration of methylene blue after traumatic brain injury diminishes neurological deficit, blood-brain barrier disruption and decrease in the expression of S100 protein in rats. Brain Research, 1740. https://doi.org/10.1016/j.brainres.2020.146854
- Hart, E. E., Blair, G. J., O’Dell, T. J., Blair, H. T., & Izquierdo, A. (2019). Anterior cingulate cortex activity regulates effort- based decision making. BioRxiv, 005, 792069. https://doi.org/10.1101/792069
- Watson, S. A., Duff, J., Bardi, I., Zabielska, M., Atanur, S. S., Jabbour, R. J., … Terracciano, C. M. (2019). Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nature Communications, 10(1), 2168. https://doi.org/10.1038/s41467-019-10175-3
- Larsen, B. R., Stoica, A., & MacAulay, N. (2019). Developmental maturation of activity-induced K + and pH transients and the associated extracellular space dynamics in the rat hippocampus. Journal of Physiology, 597(2), 583–597. https://doi.org/10.1113/JP276768
- Sánchez-Rodríguez, I., Djebari, S., Temprano-Carazo, S., Vega-Avelaira, D., Jiménez-Herrera, R., Iborra-Lázaro, G., … Navarro-López, J. D. (2019). Hippocampal long-term synaptic depression and memory deficits induced in early amyloidopathy are prevented by enhancing G-protein-gated inwardly rectifying potassium channel activity. Journal of Neurochemistry. https://doi.org/10.1111/jnc.14946
- Brown, A. G., Thapa, M., Hooker, J. W., & Ostrowski, T. D. (2019). Impaired chemoreflex correlates with decreased c-Fos in respiratory brainstem centers of the streptozotocin-induced Alzheimer’s disease rat model. Experimental Neurology, 311, 285–292. https://doi.org/10.1016/j.expneurol.2018.10.012
- Bartley, A. F., Abiraman, K., Stewart, L. T., Hossain, M. I., Gahan, D. M., Kamath, A. V., … Dobrunz, L. E. (2019). LSO:Ce Inorganic Scintillators Are Biocompatible With Neuronal and Circuit Function. Frontiers in Synaptic Neuroscience, 11. https://doi.org/10.3389/fnsyn.2019.00024
- Pickford, J., Apps, R., & Bashir, Z. I. (2019). Muscarinic Receptor Modulation of the Cerebellar Interpositus Nucleus In Vitro. Neurochemical Research, 44(3), 627–635. https://doi.org/10.1007/s11064-018-2613-9
- Li, J., & Baccei, M. L. (2019). Neonatal Injury Alters Sensory Input and Synaptic Plasticity in GABAergic Interneurons of the Adult Mouse Dorsal Horn. The Journal of Neuroscience, 39(40), 7815–7825. https://doi.org/10.1523/jneurosci.0509-19.2019
- Schappacher, K. A., Xie, W., Zhang, J. M., & Baccei, M. L. (2019). Neonatal vincristine administration modulates intrinsic neuronal excitability in the rat dorsal root ganglion and spinal dorsal horn during adolescence. Pain, 160(3), 645–657. https://doi.org/10.1097/j.pain.0000000000001444
- Ou, Q., Jacobson, Z., Abouleisa, R. R. E., Tang, X.-L., Hindi, S. M., Kumar, A., … Mohamed, T. M. A. (2019). Physiological Biomimetic Culture System for Pig and Human Heart Slices. Circulation Research, 125(6), 628–642. https://doi.org/10.1161/circresaha.119.314996
- Díaz-García, C. M., Lahmann, C., Martínez-François, J. R., Li, B., Koveal, D., Nathwani, N., … Yellen, G. (2019). Quantitative in vivo imaging of neuronal glucose concentrations with a genetically encoded fluorescence lifetime sensor. Journal of Neuroscience Research, 97(8), 946–960. https://doi.org/10.1002/jnr.24433
- Mohammed, U., Caine, R. S., Atkinson, J. A., Harrison, E. L., Wells, D., Chater, C. C., … Murchie, E. H. (2019). Rice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-41922-7
- Galloni, A. R., Laffere, A., & Rancz, E. A. (2019). Apical length modulates dendritic excitability in L5 pyramidal neurons.
- BioRxiv, 754499. https://doi.org/10.1101/754499
- Morris, P. G., Mishina, M., & Jones, S. (2018). Altered Synaptic and Extrasynaptic NMDA Receptor Properties in Substantia Nigra Dopaminergic Neurons From Mice Lacking the GluN2D Subunit. Frontiers in Cellular Neuroscience, 12. https://doi.org/10.3389/fncel.2018.00354
- Zhao, J., Baudry, M., & Jones, S. (2018). Calpain inhibition reduces NMDA receptor rundown in rat substantia nigra dopamine neurons. Neuropharmacology, 137, 221–229. https://doi.org/10.1016/j.neuropharm.2018.05.003
- Kammel, L. G., Wei, W., Jami, S. A., Voskuhl, R. R., & O’Dell, T. J. (2018). Enhanced GABAergic Tonic Inhibition Reduces Intrinsic Excitability of Hippocampal CA1 Pyramidal Cells in Experimental Autoimmune Encephalomyelitis. Neuroscience, 395, 89–100. https://doi.org/10.1016/j.neuroscience.2018.11.003
- Brewer, C. L., & Baccei, M. L. (2018). Enhanced Postsynaptic GABA B Receptor Signaling in Adult Spinal Projection Neurons after Neonatal Injury. Neuroscience, 384, 329–339. https://doi.org/10.1016/j.neuroscience.2018.05.046
- Kawatani, M., Yamada, Y., & Kawatani, M. (2018). Glucagon-like peptide-1 (GLP-1) action in the mouse area postrema neurons. Peptides, 107, 68–74. https://doi.org/10.1016/j.peptides.2018.07.010
- Murakami, T., Enjoji, M., & Koyama, S. (2018). Leptin attenuates D 2 receptor-mediated inhibition of putative ventral tegmental area dopaminergic neurons. Physiological Reports, 6(7). https://doi.org/10.14814/phy2.13631
- Ford, N. C., Ren, D., & Baccei, M. L. (2018). NALCN channels enhance the intrinsic excitability of spinal projection neurons. Pain, 159(9), 1719–1730. https://doi.org/10.1097/j.pain.0000000
- Khodai, T., Nunn, N., Worth, A. A., Feetham, C. H., Belle, M. D. C., Piggins, H. D., & Luckman, S. M. (2018). PACAP neurons in the ventromedial hypothalamic nucleus are glucose inhibited and their selective activation induces hyperglycaemia. Frontiers in Endocrinology, 9(OCT). https://doi.org/10.3389/fendo.2018.00632
- Saylor, D. M., Craven, B. A., Chandrasekar, V., Simon, D. D., Brown, R. P., & Sussman, E. M. (2018). Predicting patient exposure to nickel released from cardiovascular devices using multi-scale modeling. Acta Biomaterialia, 70, 304–314. https://doi.org/10.1016/j.actbio.2018.01.024
- Morris, G., Brennan, G. P., Reschke, C. R., Henshall, D. C., & Schorge, S. (2018). Spared CA1 pyramidal neuron function and hippocampal performance following antisense knockdown of microRNA-134. Epilepsia, 59(8), 1518–1526. https://doi.org/10.1111/epi.14475
- Jiang, L., Wang, Y., Xu, Y., Ma, D., & Wang, M. (2018). The Transient Receptor Potential Ankyrin Type 1 Plays a Critical Role in Cortical Spreading Depression. Neuroscience, 382, 23–34. https://doi.org/10.1016/j.neuroscience.2018.04.025
- Murakami, T., Enjoji, M., & Koyama, S. (2018). Leptin attenuates D 2 receptor-mediated inhibition of putative ventral tegmental area dopaminergic neurons. Physiological Reports, 6(7). https://doi.org/10.14814/phy2.13631
- Romer, S. H., Seedle, K., Turner, S. M., Li, J., Baccei, M. L., & Crone, S. A. (2017). Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Experimental Neurology, 287, 192–204. https://doi.org/10.1016/j.expneurol.2016.05.033
- Morris, G., Leite, M., Kullmann, D. M., Pavlov, I., Schorge, S., & Lignani, G. (2017). Cellular/Molecular Activity Clamp Provides Insights into Paradoxical Effects of the Anti-Seizure Drug Carbamazepine. Soc Neuroscience. https://doi.org/10.1523/JNEUROSCI.3697-16.2017
- Perbellini, F., Liu, A. K. L., Watson, S. A., Bardi, I., Rothery, S. M., & Terracciano, C. M. (2017). Free-of-Acrylamide SDS- based Tissue Clearing (FASTClear) for three dimensional visualization of myoca
- Tae, H. S., Smith, K. M., Phillips, A. M., Boyle, K. A., Li, M., Forster, I. C., … Reid, C. A. (2017). Gabapentin modulates HCN4 channel voltage-dependence. Frontiers in Pharmacology, 8(AUG). https://doi.org/10.3389/fphar.2017.00554
- Watson, & Samuel. (2017). Preparation of viable adult ventricular myocardial slices from large and small mammals.
- Nature.Com. https://doi.org/10.1038/nprot.2017.139
- Kang, C., Qiao, Y., Li, G., Baechle, K., Camelliti, P., Rentschler, S., & Efimov, I. R. (2016). Human Organotypic Cultured Cardiac Slices: New Platform For High Throughput Preclinical Human Trials. Sc
- Blot, A., de Solages, C., Ostojic, S., Szapiro, G., Hakim, V., & Léna, C. (2016). Time-invariant feed-forward inhibition of Purkinje cells in the cerebellar cortex in vivo. Journal of Physiology, 594(10), 2729–2749. https://doi.org/10.1113/JP271518
- Hablitz, L. M., Molzof, H. E., Abrahamsson, K. E., Cooper, J. M., Prosser, R. A., Karen, X., & Gamble, L. (2015). Systems/Circuits GIRK Channels Mediate the Nonphotic Effects of Exogenous Melatonin. Soc Neuroscience. https://doi.org/10.1523/JNEUROSCI.1597-15.2015
- Larsen, B. R., Assentoft, M., Cotrina, M. L., Hua, S. Z., Nedergaard, M., Kaila, K., … Macaulay, N. (2014). Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. GLIA, 62(4), 608–622. https://doi.org/10.1002/glia.22629
- Schoenberger, M., Damijonaitis, A., Zhang, Z., Nagel, D., & Trauner, D. (2014). Development of a New Photochromic Ion Channel Blocker via Azologization of Fomocaine. ACS Publications, 5(7), 514–518. https://doi.org/10.1021/cn500070w
- Yeoh, J. W., James, M. H., Graham, B. A., & Dayas, C. V. (2014). Electrophysiological characteristics of paraventricular thalamic (PVT) neurons in response to cocaine and cocaine- and amphetamine-regulated transcript (CART). Frontiers in Behavioral Neuroscience, 8(AUG). https://doi.org/10.3389/fnbeh.2014.00280
- Delerue, F., White, M., & Ittner, L. M. (2014). Inducible, tightly regulated and non-leaky neuronal gene expression in mice. Transgenic Research, 23(2), 225–233. https://doi.org/10.1007/s11
- Li, J., & Baccei, M. L. (2014). Neonatal tissue injury reduces the intrinsic excitability of adult mouse superficial dorsal horn neurons. Neuroscience, 256, 392–402. https://doi.org/10.1016/j.neuroscience.2013.10.057
- Guilmi, M. N. Di, Wang, T., Gonzalez Inchauspe, C., Forsythe, I. D., Ferrari, M. D., Van Den Maagdenberg, A. M. J. M., … Uchitel, O. D. (2014). Neurobiology of Disease Synaptic Gain-of-Function Effects of Mutant Ca v 2.1 Channels in a Mouse Model of Familial Hemiplegic Migraine Are Due to Increased Basal [Ca 2 ] i. Soc Neuroscience. https://doi.org/10.1523/JNEUROSCI.2526-13.2014
- Xiong, W., Chen, S. R., He, L., Cheng, K., Zhao, Y. L., Chen, H., … Zhang, L. (2014). Presynaptic glycine receptors as a potential therapeutic target for hyperekplexia disease. Nature Neuroscience 17, 232-239. https://doi.org/10.1038/nn.3615
- Hablitz, L. M., Molzof, H. E., Paul, J. R., Johnson, R. L., & Gamble, K. L. (2014). Suprachiasmatic nucleus function and circadian entrainment are modulated by G protein-coupled inwardly rectifying (GIRK) Channels. J. Physiol. 592.22, 5079-5092. https://doi.org/10.1113/jphysiol.2014.282079
- Cameron, M. A., Suaning, G. J., Lovell, N. H., & Morley, J. W. (2013). Electrical Stimulation of Inner Retinal Neurons in Wild-Type and Retinally Degenerate (rd/rd) Mice. PLoS ONE, 8(7). https://doi.org/10.1371/journal.pone.0068882
- Wild, A. R., Akyol, E., Brothwell, S. L. C., Kimkool, P., Skepper, J. N., Gibb, A. J., & Jones, S. (2013). Memantine block depends on agonist presentation at the NMDA receptor in substantia nigra pars compacta dopamine neurones. Neuropharmcology 73, 138 – 146. https://doi.org/10.1016/j.neuropharm.2013.05.013
- Koyama, S., Kawaharada, M., Terai, H., Ohkurano, M., Mori, M., Kanamaru, S., & Hirose, S. (2013). Obesity decreases excitability of putative ventral tegmental area GABAergic neurons. Physiological Reports 1(5), 1-11 https://doi.org/10.1002/phy2.126
- Huang, S., & Uusisaari, M. Y. (2013). Physiological temperature during brain slicing enhances the quality of acute slice preparations Frontiers Neuroscience, 7.
- https://www.frontiersin.org/articles/10.3389/fncel.2013.00048/full
- Dougherty, S. E., Reeves, J. L., Lesort, M., Detloff, P. J., & Cowell, R. M. (2013). Purkinje cell dysfunction and loss in a knock-in mouse model of Huntington Disease. Experimental Neurology, 240(1), 96–102. https://doi.org/10.1016/j.expneurol.2012.11.015
- Dougherty, S. E., Reeves, J. L., Lesort, M., Detloff, P. J., & Cowell, R. M. (2013). Purkinje cell dysfunction and loss in a knock-in mouse model of Huntington Disease. Experimental Neurology,
- Puskarjov, M., Ahmad, F., Kaila, K., & Blaesse, P. (2012). Development/Plasticity/Repair Activity-Dependent Cleavage of the K-Cl Cotransporter KCC2 Mediated by Calcium-Activated Protease Calpain. Soc Neuroscience. https://doi.org/10.1523/JNEUROSCI.6265-11.2012
- Mewes, A., Franke, H., & Singer, D. (2012). Organotypic Brain Slice Cultures of Adult Transgenic P301S Mice-A Model for Tauopathy Studies. PLoS ONE, 7(9). https://doi.org/10.1371/journal.pone.0045017


