Controlled substance inventory system for freezers, refrigerators, cabintes
Efficient management of controlled substances in the animal research facility.
The use of controlled substances in the laboratory animal research facility is strictly regulated. Laboratory and veterinary staff who store and utilize controlled substances at their facilities must comply with all applicable State and Federal laws and regulations. Authorized agents are directly responsible for the storage and security and of all controlled substances on site, and they find it increasingly difficult to manage the process effectively, which includes maintaining accurate and detailed inventory records to ensure compliance.
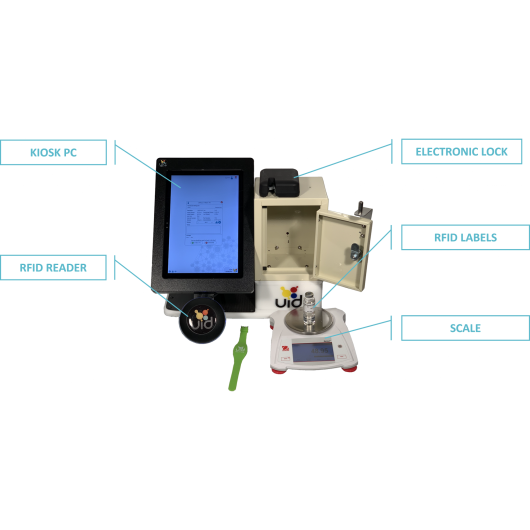
The UID Controlled Substance Inventory System (CSI-360) was specifically designed to improve security, ensure compliance and facilitate the management of controlled substances. UID solution focuses on diversion control, where other systems merely dispense the drugs. The CSI-360 combines a two-part authentication process, novel software and RFID tracking technology to provide secure access and 100% accurate recording of all controlled substance use in the animal research facility. All records are captured electronically for a precise account of all cabinet access and activity, saving time and eliminating data entry errors.
Compatible with all cabinets, safes, refrigerators, and freezers from most manufacturers
UDI controlled substance inventory system solution is designed to integrate with existing narcotics lock boxes to eliminate the painful process of DEA recertification. UID provides an electronic locking mechanism, RFID technology for secure user access and asset identification (fob, key card or wrist band; RFID scanner and labels), a digital scale for precise measurements, a Windows Surface computer and inventory management software to enable efficient tracking and recordkeeping for controlled substances.
Unique capabilities controlled substance inventory system
- Secure access with two-factor validation
- Authorize or restrict user access
- Accurate inventory of all contents
- Paperless recording for accurate documentation
- Automatic and electronic tracking from acquisition to administration
- Detailed reports: inventory, activity, user access and more
- All documents are easily retrievable and readily available for dea audits
- Network multiple cabinets