Vivarium - Why qualify the equipment and what standards?
For Quality management, the standard that is applicable to pre-clinical regulatory studies is Good Laboratory Practices (GLP).
Within this specific context, their aim is to promote reliable and high-quality studies. Even though they are not applicable in regulatory terms outside pre-clinical development studies, they are particularly useful as an approach and methodology for all scientific studies. They have been generalised under the term “Good Scientific Practices” to ensure that all studies are reliable and repeatable. One component of GLP relates to resources and means (including facilities and equipment).
In another field, that of biological risk management, there is a requirement to ensure that containment systems are effective through well-defined tests, in line with the nature of the risk and on the basis of appropriate acceptance criteria. “Systems qualification” is essential to demonstrate the relevance and efficacy of equipment and practices in accordance with the expected results.
The "Guide for the Care and Use of Laboratory Animals", which is the primary standard for AAALAC accreditation, requires that washing equipment must be assessed periodically to ensure that it is in good working order and is being used properly in order to guarantee the expected result, objectivised with appropriate methods and indicators.
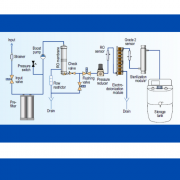
In addition to the other prior stages (Design Qualification – DQ, “Factory Acceptance Test” – FAT, and “Site Acceptance Test” – SAT), a validation protocol must describe the qualification of the installation and the equipment, the objectives, the field of application and the different phases, namely: Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ).
IQ aims to provide documented evidence that the equipment complies with the client’s requirements (specifications) and the manufacturer’s specifications: installation, connections, measuring instruments, etc. The purpose of OQ is to check that the equipment, its control system and its instrumentation work within the required parameters: regulation, safety, precision and adequacy of the parameters of the operating cycle. These stages are generally completed by the supplier.
PQ makes it possible to ensure, in a documented manner, that the facilities, systems and equipment, as installed, are used and work effectively and reproducibly in accordance with the approved operational method and comply with the specifications and the expected results. For example, in a sterilisation or decontamination autoclave, the expected result must be achieved at all levels of every standard autoclave load, with appropriate physical and microbiological indicators.
A Qualification Plan comprises initial and periodic qualifications. OQ uses indicators of different kinds (cleaning indicators, thermal, chemical and biological indicators of disinfection, decontamination or sterilisation) which are appropriate for demonstrating efficacy, and an established number of indicators and positioning plan in vivarium.
Article source on Allentown website here