



Nanoliter 2020 injector
Precise nanoliter-volume injections with intuitive SMARTouch™ controller and accessories. NANOLITER2020 is the complete system for making nanoliter to microliter range injections into frogs (Xenopus oocytes), rats, mice, mosquitoes, shrimps, insects (e.g., Brown Planthopper), flies (e.g., Drosophila) and fish (Zebrafish) embryo using glass micropipettes.
- Perform nanoliter volume injections using glass micropipettes
- Nanoliter2020 Injector works on the principle of Positive Displacement. An internal micrometer step motor precisely moves the metal plunger which pushes the oil inside the micropipette, and the oil-layer pushes nanoliter volumes of aqueous sample out of the pipette tip.
- Fine control of plunger displacement along with proper sealing among gasket, glass micropipette, and oil ensures precision and accuracy
- MICRO2T SMARTouch™ controller is required to operate the 300704 Injector (The same MICRO2T controller can be used with UMP3 pumps.)
- 300746 Spare Parts Kit required for use with the 300704 Injector
- Glass micropipettes are not included with the 300704. Use WPI glass micropipettes or use glass capillaries (504949 or 504950) and a puller to make micropipettes.
- Use a tweezer (501981) to scoop gaskets out of the NANOLITER2020 Injector Head
- 300704 Injector Head comes with one set of gaskets installed in the injector to use with 1.1-1.15 mm OD fire-polished glass
Benefits of NANOLITER2020 Injector (300704)
- Injections performed with mineral oil back-filled glass micropipettes, and the sample is front filled. This approach minimizes loss of any costly and scarce sample when a minute sample volume can be adequate. (See FAQ for details.)
- Control up to two pumps simultaneously
- Has capability to be easily mounted on a micromanipulator (e.g., M3301) or a stereotaxic frame (e.g. 505313) using the universal adapter 500778 (included)
- Precise control over injection volumes (in the nanoliter to microliter range) and injection rates
- Simple to use touchscreen interface and graphical representation of volume status on MICRO2T controller
- LED run indicator offers a visual indication of connectivity
- Perform the injection with a foot switch (optional)
- Injector is compatible with different OD glass micropipettes (in the range of 1.1-1.5 mm)
- NANOLITER2020 costs 15% less than the previous model (NL2010MC2T)!
Applications
Nanoliter to microliter injection into frogs (Xenopus oocytes), rats, mice, mosquitoes, shrimps, insects (e.g., Brown Planthopper), flies (e.g., Drosophila) and fish (Zebrafish) embryo using glass micropipettes
- 300704 NANOLITER2020 Injector Head [One Set of Gasket (Front Green Gasket) to use with 1.1-1.15 mm Glass is installed]
- MICRO2T SMARTouch Controller
- 501981 Tweezers to scoop out gaskets
- 504949 Glass capillary (ID = 0.530 mm, OD 1.14 mm), 1 pack of 300 psc.
- 1TIP10XV119 Glass micropipettes (ID = 0.530 mm, OD 1.14 mm, Tip ID= 10 μm)
- ----------------------------------------------------------------------------------------
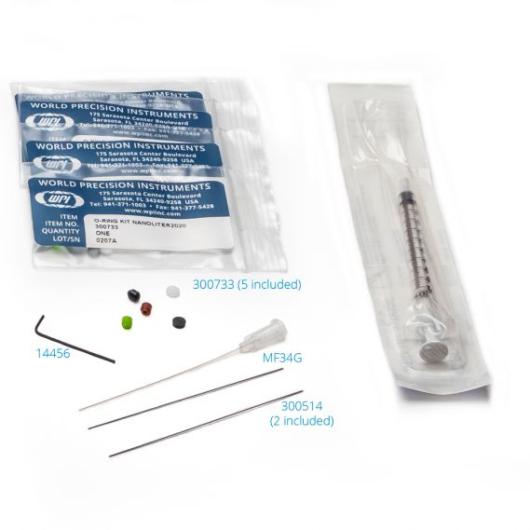
- 300746 SPARE PART KIT include:
- 14456 Allen Wrench 0.035 Hex Tool
- MF34G MicroFil 34 ga
- 300703 O-Ring Kit for NANOLITER2020, 5 psc.
- 3563 1 cc Syringe
- 300514 Replacement Piston for NANOLITER2020, 2 psc.
Teng S, Peng Y. Simultaneous Microendoscopic Calcium Imaging and EEG Recording of Mouse Brain during Sleep.
Bio Protoc. 2023 May 5;13(9):e4664. doi: 10.21769/BioProtoc.4664. PMID: 37188105; PMCID: PMC10176210.
Gaete, P., Lillo, M., López, W., Liu, Y., Harris, A., & Contreras, J. (2020).
A novel voltage clamp/dye uptake assay reveals saturable transport of molecules through CALHM1 and connexin channels.
BioRxiv, 2020.02.15.950923. https://doi.org/10.1101/2020.02.15.950923
Atif, M., Lynch, J. W., & Keramidas, A. (2020). The effects of insecticides on two splice variants of the glutamate-gated chloride channel receptor of the major malaria vector,
Anopheles gambiae . British Journal of Pharmacology, 177(1), 175-187. https://doi.org/10.1111/bph.14855
Hao, D.-L., Yang, S.-Y., Liu, S.-X., Zhou, J.-Y., Huang, Y.-N., Véry, A.-A., … Su, Y.-H. (2020). Functional Characterization of the Arabidopsis Ammonium Transporter AtAMT1;3 With the Emphasis on Structural Determinants of Substrate Binding and Permeation Properties.
Frontiers in Plant Science, 11, 571. https://doi.org/10.3389/fpls.2020.00571
Li, Y., Liu, Z., Guo, Q., & Luo, M. (2019). Long-term Fiber Photometry for Neuroscience Studies.
Neuroscience Bulletin, 35(3), 425–433. https://doi.org/10.1007/s12264-019-00379-4
Li, R., Weng, J., Wang, X., Meng, Q., Wang, Y., & Sun, J. (2019). Bursicon homodimers induce innate immune by activating the expression of anti-microbial peptide genes in the shrimp Neocaridina heteropoda.
Fish and Shellfish Immunology, 84, 906–911. https://doi.org/10.1016/j.fsi.2018.10.080
Li, R., Huang, Y., Zhang, Q., Zhou, H., Jin, P., & Ma, F. (2019). The miR-317 functions as a negative regulator of Toll immune response and influences Drosophila survival.
Developmental and Comparative Immunology, 95, 19–27. https://doi.org/10.1016/j.dci.2019.01.012
Li, X., Liu, F., Wu, C., Zhao, J., Cai, W., & Hua, H. (2019). Decapentaplegic function in wing vein development and wing morph transformation in brown planthopper, Nilaparvata lugens.
Developmental Biology, 449(2), 143–150. https://doi.org/10.1016/j.ydbio.2019.02.016
Scala, R., Maqoud, F., Angelelli, M., Latorre, R., Perrone, M., Scilimati, A., & Tricarico, D. (2019). Zoledronic Acid Modulation of TRPV1 Channel Currents in Osteoblast Cell Line and Native Rat and Mouse Bone Marrow-Derived Osteoblasts: Cell Proliferation and Mineralization Effect.
Cancers, 11(2), 206. https://doi.org/10.3390/cancers11020206